Antibodies are an indispensable reagent in biotechnology, supporting applications in basic research, diagnostic and therapeutic areas.
While traditional hybridoma technology has been successful in producing highly specific and pure antibodies, it is not without its limits. Genetic drift can occur when the nucleic acid sequence of the antibody-encoding genes changes over time as the hybridoma cells divide. Recombinant antibodies provide an alternative that overcomes these limitations and ensures reproducibility, as in vitro synthetic genes are employed to produce recombinant antibodies.
In this blog, we examine the benefits of recombinant antibodies and discuss how the Chinese Hamster Ovary (CHO) mammalian cell line is optimized for recombinant antibody production.
Antibodies are a natural defense against invading pathogens. They are naturally produced by plasma cells within the human body. Antibodies are also critical reagents in the biotechnology industry, with several applications in therapy and immunomodulation, diagnostic testing and vaccines.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
The majority of therapeutic monoclonal antibodies utilize the hybridoma technique developed by Köhler and Milstein in 19751. Here, B-lymphocytes are isolated from mice following immunization with an antigen and consequently fused with immortal myeloma cells to form hybridoma cells, which can be cultured to produce monoclonal antibodies.
Recombinant antibody production, a method that circumvents the use of animals and offers significant economic benefits, is a viable alternative for large-scale antibody production. This method provides a sustainable solution that surpasses in vivo antibody production in terms of reproducibility and ensures that the antibodies are precisely defined at a sequence level.
Over the years, the production of monoclonal antibodies has shifted from a reliance on hybridoma cell lines to recombinant technologies. The recombinant antibody production process is performed in vitro, with the cloning and expression of synthetic antibody genes in a suitable host. Mammalian cell culture is the preferred expression system as it can produce full-length antibodies with the correct post-translational modifications and folding, which results in antibodies with functional biological activity and low immunogenicity.
Mammalian cells are commonly chosen over non-mammalian cells (such as bacteria cells), with the majority of recombinantly produced approved products made in mammalian cells2. Commonly used mammalian cell lines are Chinese Hamster Ovary (CHO) cells, NS0, Sp2/0t, HEK293, and PER.C6 cells. CHO cells are the dominating production system for recombinant mAbs, accounting for 70% of the production of biopharmaceutical proteins, most of which are monoclonal antibodies3. There are numerous reasons why CHO cells remain the most commonly used cell line in bioprocessing.
CHO cell production has generated therapeutic antibodies for diseases such as cancer, multiple sclerosis, asthma, HIV, and neuroblastoma. The advantages of employing CHO cells include:
Recombinant antibody production can be broadly divided into four main steps:
Step 1: Protein sequence identification: the sequence of the desired protein is obtained
Step 2: Gene expression
Step 3: Transfection into CHO cells and optimization of production
Step 4: Purification of the produced antibody
CHO cells have been optimized to maximize protein production. Selection marker genes in the expression vectors can be used to select cells, and selection drugs can amplify genes following transfection with expression vectors. One of the most widely used is the CHO-Dhfr expression system. DHFR reduces dihydrofolate to tetrahydrofolate, which is involved in purine synthesis.
DHFR-deficient CHO host cells cannot survive in media lacking purine or grow in media lacking hypoxanthine (or adenine), glycine, and thymidine (GHT). The clonal selection allows the expression of the DHFR gene, which restores the cell’s ability to grow in GHT media. Amplification of the integrated gene can be achieved using increasing concentrations of methotrexate (MTX), which inhibits DHFR with high affinity and thus increases selection pressure to favor the high expression of the desired gene.
Clonal selection and gene amplification can also be achieved in a similar manner by employing CHO cells with glutathione synthetase (GS) deficiency. GS-deficient cells cannot catalyse the production of glutamine from glutamate and ammonia, which is crucial for replication and growth. GS can be reintroduced following transfection with a vector containing gs and mAb genes. Further amplification can be achieved using the GS inhibitor methionine sulfoximine (MSX), which produces high product yields.
The most important carbon and nitrogen sources in cell culture media are glucose and glutamine. However, more slowly metabolized compounds are preferred as they avoid the buildup of lactate and ammonia and ensure cell growth. CHO cells are highly adaptable to changes in medium composition, meaning glucose and glutamine could be substituted with galactose and glutamate9. Media optimization is essential for scaling up production from small-scale to larger scale using bioreactors. Modification of transcriptional and post-transcriptional pathways in CHO cells has also been shown to result in increased titres10.
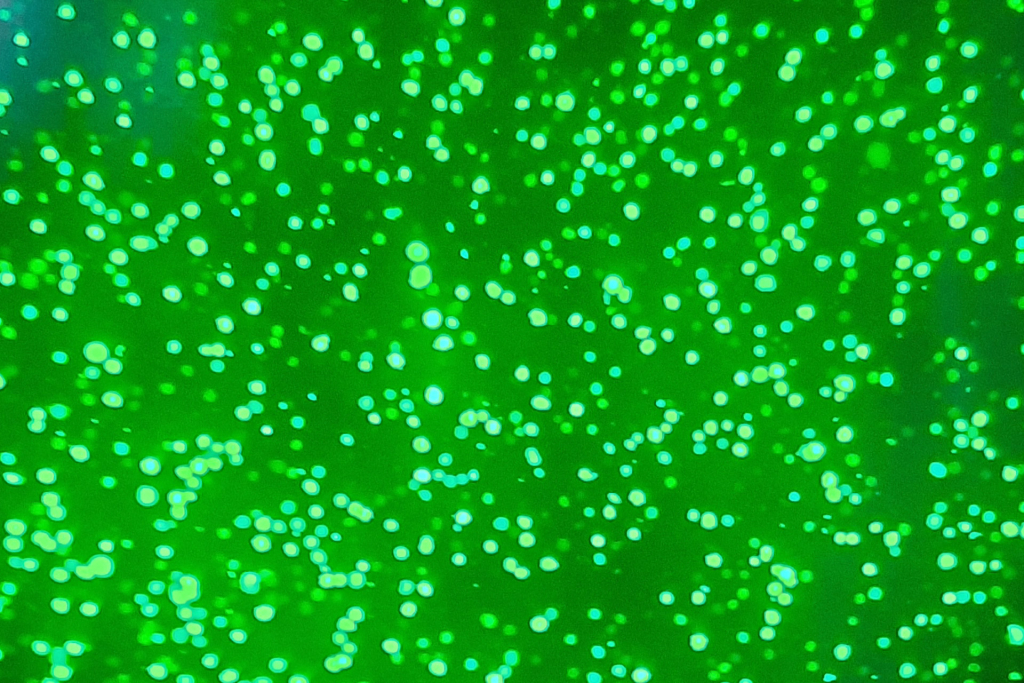
Figure 1. Fluorescence microscopy of CHO cells transfected with an eGFP gene
CHO cells have come a long way from the first production of insulin to producing the majority of biotherapeutics in the USA and EU today. The number of new biotherapeutics like viral antigens, vaccines, and bi- and tri-specific antibodies derived from CHO cell cultures has increased, which means we are excited to see what the future holds for CHO cell antibody production. Here at evitria, we are convinced that new, revolutionary developments lie ahead of us.
At evitria, we provide recombinant antibody expression services for both commercial and smaller-scale applications, with a particular focus on in vitro CHO cell culture-based production. Our technology leverages the advantages of CHO cells described in this article, allowing us to eliminate the need for laboratory animals and their immunization, resulting in no animal contaminants in our recombinant antibodies.
The antibody production services at evitria offer faster turnaround times and greater yields than conventional methods. Our expertise includes the production of various recombinant antibodies in CHO cells, including chimeric, bispecific, Fc-optimized and fusion antibodies, afucosylated antibodies, and Fc-silenced antibodies.
Advantages of evitria’s recombinant antibody production service include high expression levels, stability, and the ability to perform glyco-engineering. As part of the service, a range of purification methods – from affinity chromatography using protein A and other resins – are employed to ensure a highly purified final product that is absent of impurities and conforms with regulatory guidelines. Our antibody production services produce antibodies for research, therapeutic, and diagnostic applications.
Several types of cells can be used for antibody production, but the most common are mammalian cells, such as Chinese Hamster Ovary (CHO) cells, HEK293 cells, and NS0 cells. CHO cells are the most widely used for producing recombinant monoclonal antibodies due to their ability to perform post-translational modifications on antibody structures, resulting in improved antibody stability and efficacy and high yields of recombinant proteins.